Introduction: Venetoclax (Ven) is an orally administered, highly selective inhibitor of B-cell lymphoma-2 (BCL-2). Fixed-duration VenR improved outcomes versus standard bendamustine-rituximab (BR) in the randomized Phase III MURANO study (NCT02005471; Seymour et al. N Engl J Med 2018) and is now a standard of care for the treatment of pts with R/R CLL. There are currently limited data to guide subsequent therapies when relapse occurs after fixed-duration VenR and uncertainty regarding the efficacy of repeated VenR treatment. Here, we report the response rates to follow-up therapy with Ven and Ven-based regimens, or exposure to Bruton tyrosine kinase inhibitor (BTKi) salvage therapy, following pts' participation in the MURANO trial.
Methods: Pts were randomized to VenR (Ven 400 mg daily for 2 years [yrs] plus monthly R for the first 6 months [mo]) or BR (6 mo). The primary endpoint was investigator-assessed progression-free survival (PFS). Pts in either arm with disease progression were followed for overall survival (OS) and disease response to any subsequent anti-CLL therapeutic regimens. Pts who initiated new anti-CLL therapy, but who had not had a response assessment reported by the principal investigator, were considered unevaluable.
Results: 389 pts were enrolled in MURANO (VenR, n=194; BR, n=195). At a clinical cutoff date of May 8, 2020, all main study pts had ceased treatment with a median follow-up of 59 mo (range 0-71.5). PFS and OS benefits were maintained at the 5-yr follow-up (Kater et al. Submitted to ASH 2020).
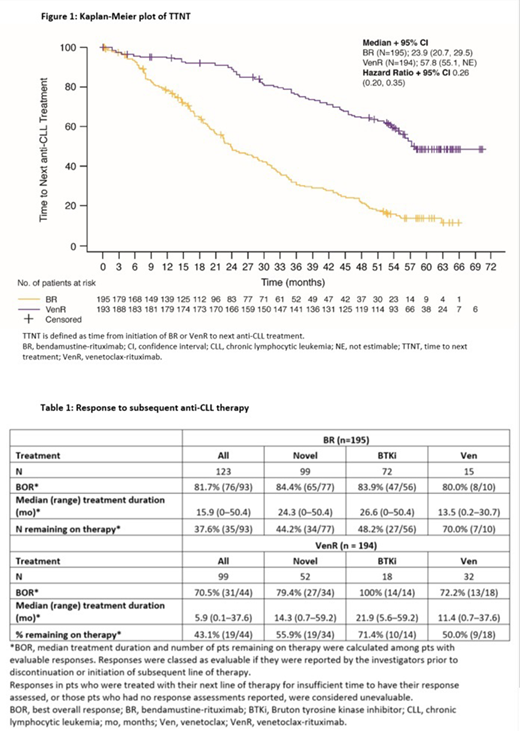
Following disease progression, 67/87 (77.0%) VenR pts and 123/148 (83.1%) BR pts had received subsequent anti-CLL therapy. The time to next therapy (TTNT) from study entry was longer following VenR versus BR, with a median TTNT of 57.8 (95% CI: 55.1-NE) mo versus 23.9 (95% CI: 20.7-29.5) mo (HR, 0.26 [95% CI: 0.20-0.35]; p<0.001), respectively (Figure 1). Best overall response (BOR) rates to first subsequent anti-CLL therapy for pts with evaluable responses were 70.5% for VenR compared with 81.7% for BR.
Of the BR pts receiving subsequent therapy; 99/123 (80.5%) pts received novel targeted therapy alone or in combination with other agents (BTKi, n=72; phosphoinositide 3-kinase inhibitors [PI3Ki], n=10; Ven, n=15; or other investigational medicinal products [IMP], n=2) while the remaining 24 pts received chemoimmunotherapy. Of pts previously treated with BR, the BOR rate to novel targeted agents was 84.4% among evaluable pts (83.9% for BTKi and 80.0% for Ven-based therapy; Table 1).
Fifty two of 67 pts in the VenR arm received subsequent novel therapy (BTKi, n=18; PI3Ki, n=1; Ven [alone or in combination], n=32; IMP, n=1). The BOR rate to these targeted agents was 79.4% among evaluable pts. After a median treatment-free interval of 13.5 (range 0.0-41.3) mo, 18 VenR pts received a BTKi as their next line of therapy (all were BTKi naïve). These pts achieved high overall response rates (ORR): 14/14 (100% of pts with an evaluable assessment) at a median treatment duration of 21.9 (range 5.6-59.2) mo, with 10 pts continuing on BTKi therapy at this follow-up.
After a median treatment-free interval of 23.7 (range 3.3-43.8) mo, 32 VenR pts were re-treated with Ven-based regimens; 21 were enrolled in the re-treatment arm of the MURANO sub-study and 11 were treated outside of the sub-study. The BOR to re-treatment with Ven or Ven-containing therapies was 72.2% of evaluable pts (Table 1). Among these pts, initial response to VenR at the main study end of combination treatment response visit was 100% (6 complete response [CR]/CR with incomplete hematologic recovery; 12 partial response), with 77.8% (14/18) achieving undetectable minimal residual disease and 15/18 completing the initial 2 yrs of Ven therapy without progression. Median treatment duration in evaluable pts re-treated with Ven-based regimens was 11.4 (range 0.7-37.6) mo with 50% of pts continuing on therapy.
Conclusions: Five-yr data from MURANO demonstrated sustained TTNT benefit with VenR versus BR. Despite >80% of relapsed BR pts receiving salvage therapy with a novel agent, OS rates remain superior with VenR therapy. Relapsed VenR pts demonstrated high ORR to either subsequent BTKi therapy or re-exposure to Ven-based regimens. These data show early use of fixed-duration VenR in R/R CLL is an effective approach and does not compromise subsequent therapy response or OS.
Owen:AbbVie, F. Hoffmann-La Roche, Janssen, Astrazeneca, Merck, Servier, Novartis, Teva: Honoraria. D'Rozario:AbbVie: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Robak:GSK: Research Funding; Bristol Meyers Squibb: Research Funding; Medical University of Lodz: Current Employment; Morphosys: Research Funding; Takeda: Consultancy; UCB: Honoraria, Research Funding; Octapharma: Honoraria; AbbVie: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Acerta: Research Funding; Roche: Consultancy, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Novartis: Honoraria, Research Funding; Sandoz: Consultancy, Honoraria; UTX-TGR: Research Funding; Momenta: Consultancy; Pfizer: Research Funding; AstraZeneca: Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; BioGene: Honoraria, Research Funding. Kater:Celgene, F. Hoffmann-La Roche/Genentech, Astra Zeneca, Janssen: Honoraria; Celgene, F. Hoffmann-La Roche/Genentech, Astra Zeneca, Janssen: Research Funding. Montillo:AbbVie: Honoraria, Speakers Bureau; F. Hoffmann-La Roche: Honoraria, Research Funding; Janssen: Honoraria, Speakers Bureau; Astra Zeneca: Honoraria; Gilead: Honoraria, Speakers Bureau; Verastem: Honoraria. de la Serna:Abbvie, AstraZeneca: Other: Travel, Accommodations, Expenses; Abbvie, Pharmacyclics, Novartis, Janssen, Acerta, AstraZeneca, BioGene, UCB, Sandoz: Honoraria; Gilead, AstraZeneca, Abbvie, Janssen, Sandoz, F. Hoffmann-La Roche: Consultancy; Abbvie, Janssen: Speakers Bureau; F. Hoffmann-La Roche, Abbvie, Pharmacyclics, Gilead, GlaxoSmithKline, Novartis, Janssen, Roche, Acerta, AstraZeneca, BioGene, UCB: Research Funding. Trněný:Bristol Meyers Squibb: Consultancy, Honoraria, Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company); Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other: TRAVEL, ACCOMODATIONS, EXPENSES (paid by any for-profit health care company); AbbVie: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Incyte: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Gilead Sciences: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); 1st Faculty of Medicine, Charles University, General Hospital in Prague: Current Employment; Roche: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Celgene: Consultancy. Kim:AbbVie, Inc.: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Other: may hold stock or other options. Bataillard:Imperial College Healthcare NHS Trust: Ended employment in the past 24 months; Roche Products Limited (temporary clinical fellowship as a fixed-term sabbatical from Hematology specialty training fellowship at Imperial College Healthcare NHS Trust): Current Employment. Lefebure:F. Hoffmann-La Roche: Current Employment, Current equity holder in publicly-traded company. Boyer:Roche: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Seymour:Mei Pharma: Consultancy, Honoraria; F. Hoffmann-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy; Nurix: Honoraria; Morphosys: Consultancy, Honoraria; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal